Regulation
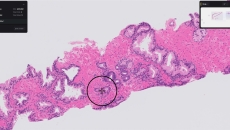
Paige Prostate analyzes digitized biopsy slides and identifies areas that could be cancerous for the pathologist to review further.
Telehealth made up 4.5% of medical claim lines in June, falling from 5% in May.
The company develops AI tools to analyse chest x-rays and breast mammography.
All students over the age of 12 and school staff members must be fully vaccinated in order to be allowed to attend school, the Kingdom’s Ministry of Education said.
The company is also subject to a class action litigation and personal injury claims.
The small study found nearly all patients had a complete abortion without the need for intervention.
HIMSS21
HIMSS Senior VP of Government Relations Tom Leary provides updates on Patient ID Now, pandemic response and the telehealth cliff at HIMSS21.
With this designation, Swing is launching a real-world clinical trial to assess its fibromyalgia DTx.
HIMSS21
“I think those APIs for consumers will be as transformative as APIs have been for music, in printing, in travel, in banking and retail,” said Dr. Don Rucker, former ONC National Coordinator for Health IT, at a HIMSS21 lightning session.
Abbott has received clearance from the FDA for its imaging software that uses artificial intelligence to provide doctors a better view of blood flow and blockages in heart vessels.